When it comes to protein analysis, two of the most commonly used techniques in biochemistry and molecular biology laboratories are 2D gel electrophoresis and SDS PAGE. These methods play crucial roles in protein separation, identification, and characterization, yet they differ in complexity, resolution, and application. Understanding their distinctions and advantages can help researchers choose the right technique for their specific needs. If you are looking for professional lab services, check over here to get expert guidance on electrophoresis techniques.
What is SDS PAGE?
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS PAGE) is a widely used method for separating proteins based on their molecular weight. It is a one-dimensional electrophoresis technique, meaning it separates proteins in a single direction through a polyacrylamide gel matrix.
How SDS PAGE Works
Protein Denaturation – Proteins are treated with SDS detergent, which denatures them and gives them a uniform negative charge.
Electrophoresis – The denatured proteins are loaded into the polyacrylamide gel and subjected to an electric field, which causes them to migrate based on their size.
Visualization – After separation, the proteins are stained using dyes such as Coomassie Brilliant Blue or silver stain for analysis.
Advantages of SDS PAGE
Simplicity – Requires fewer preparation steps than 2D electrophoresis.
Reproducibility – Provides consistent results across different runs.
High Resolution for Molecular Weight Determination – Useful for distinguishing proteins of different molecular weights.
Faster Execution – Typically takes a few hours from sample preparation to visualization.
For more details on SDS PAGE and how it fits into protein separation workflows, click this link here now.
What is 2D Gel Electrophoresis?
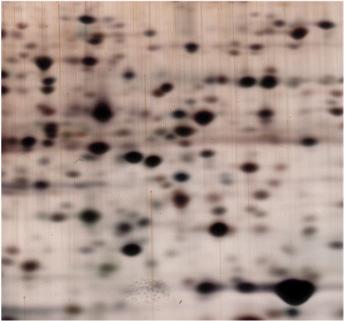
Two-dimensional gel electrophoresis (2D PAGE) is an advanced protein separation technique that separates proteins in two dimensions: isoelectric focusing (IEF) in the first dimension and SDS PAGE in the second dimension.
How 2D Gel Electrophoresis Works
Isoelectric Focusing (IEF) – Proteins are first separated by their isoelectric point (pI) using a pH gradient.
SDS PAGE Separation – The proteins from the first step are then separated by molecular weight in a polyacrylamide gel.
Staining & Analysis – Staining methods such as Coomassie Blue, silver staining, or fluorescent dyes are used to visualize the proteins.
Advantages of 2D Gel Electrophoresis
High Resolution & Separation Power – Can resolve thousands of proteins in a single experiment.
Comprehensive Protein Profiling – Useful for detecting post-translational modifications.
Effective for Complex Samples – Provides detailed information about proteomic changes in cells.
To learn more about advanced protein analysis techniques, click here to find out more.
Choosing the Right Technique for Your Needs
- If you need fast, reliable, and reproducible separation of proteins based on size, SDS PAGE is the best choice.
- If you require detailed protein profiling with high-resolution separation, 2D electrophoresis is more suitable.
- For complex biological samples with many proteins, 2D PAGE is more advantageous.
For expert consultation on which technique suits your research, Contact us today for professional lab services.
Final Thoughts
Both SDS PAGE and 2D gel electrophoresis are invaluable in protein analysis, but their applications differ based on resolution, complexity, and research goals. Whether you need quick molecular weight determination or comprehensive proteomic analysis, selecting the right method can significantly impact your experimental success.
For more information on protein separation techniques and to access state-of-the-art laboratory services, check over here to explore professional guidance.
By understanding the strengths and limitations of each method, you can optimize your experimental outcomes and achieve more precise and reliable protein analysis results.